In meiotic cells, a wave of DNA double-strand breaks is physiologically induced at meiosis onset to allow homologous recombination to take place. This in turn, elicits the formation of crossovers, which are essential for the correct partitioning of the genetic material in the haploid gametes.
Under normal conditions, a strong homologous bias ensures that meiotic breaks are repaired in a conservative and error-free fashion. However, upon perturbed regulation of DNA repair mechanisms, meiotic DSBs can be hijacked by multiple illegitimate repair pathways, which can produce toxic outcomes.
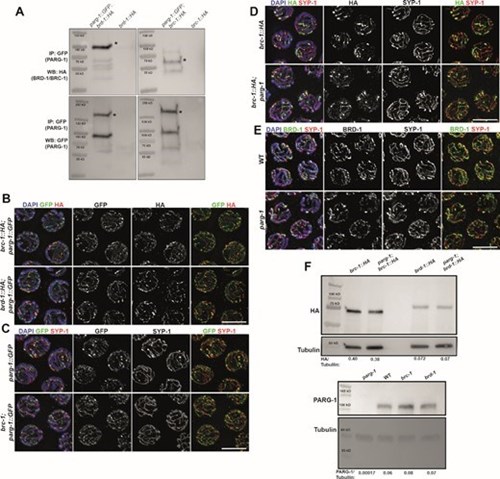
The latest research of Nicola Silva´s lab found that the combined action of two important genes involved in DNA repair, the poly(ADP-ribose) eraser PARG and the E3 ubiquitin ligase BRCA1, is essential to regulate DNA repair pathway choice during meiosis, ensuring accurate chromosome segregation.
The latest research of Nicola Silva´s lab found that the combined action of two important genes involved in DNA repair, the poly(ADP-ribose) eraser PARG and the E3 ubiquitin ligase BRCA1, is essential to regulate DNA repair pathway choice during meiosis, ensuring accurate chromosome segregation.
Mutations in BRCA1 in humans are causative of breast/ovarian cancer susceptibility. In recent years, it has been discovered that abolishing ADP-ribosylation in BRCA1-mutated cells confers high levels of cancer cells death, which is currently exploited with high success in therapy. However, over time resistance mechanisms ensue, which render treatment less effective.
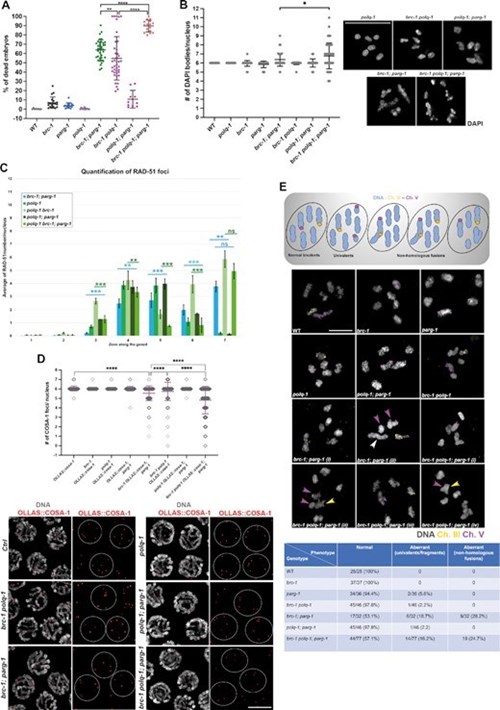
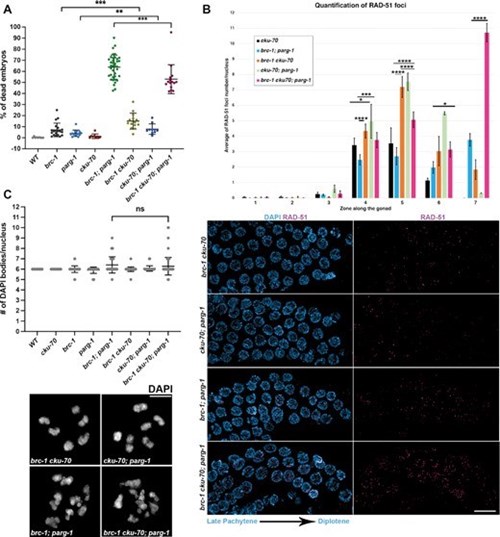
The research outcomes demonstrate that preventing ADP-ribosylation removal in BRCA1-mutated worms by abrogating PARG function causes high levels of embryonic lethality and impairs DNA repair, highlighting PARG as a putative target for future cancer therapy applications. In mammalian models, this type of research has been hindered in vivo by the embryonic lethality observed in mice when BRCA1 or PARG are mutated, further emphasizing how the use of simpler metazoan models can be instrumental to human health.
The latest results have recently been published in the prestigious journal Nucleic Acids Research